In addition to being a regulatory or statutory requirement, GMP certification is a statement to your customer, competitors, and the general public about your manufacturing practices and your commitment to the highest level of health, safety, and sanitation standards.
GMP for Cannabis CBD and Hemp clearly distinguishes the quality of your products from your competitors in the Cannabis, CBD, and Hemp industry. In some states, like New York, GMP is also a requirement to obtain licensure for cannabis, CBD, and hemp companies.
GMP AUDIT FOR CANNABIS CBD AND HEMP
Manufacturers and processors of cannabinoid products are required to implement a Quality Management System and comply with GMP requirements. GMP is enforced by various state and federal regulatory agencies. They do site audits and inspections based on relevant standards. Proof of a qualified third-party GMP audit of the applicant’s extraction and manufacturing process is required for licensure of Cannabis, CBD, and Hemp companies.
STATE GMP REGULATIONS FOR CANNABIS CBD AND HEMP
Markets for Cannabis, CBD, and Hemp products in the United States and across the world are fast growing as more and more governments are thinking about or are in the process of legalizing these products.
In the United States, the Cannabis, CBD, and Hemp industries are widely unregulated at the national level and no guidelines have been developed by the FDA about the Quality Management System requirements or GMP for the manufacturers of these products. The cannabis industry is expecting a new policy platform and federal regulations to fill the regulatory gap resulting from the lack of a national standard by the FDA. The requirements are largely regulated locally by the individual states.
For example, New York state has recently announced regulations to set quality control standards that all cannabinoid hemp processors and retailers will be required to meet in order to license their product. They must go through a third-party audit to obtain and maintain Good Manufacturing Practice (GMP) Certification referred to as “GMP for New York Cannabinoid Hemp Program.”
FDA GMP FOR CANNABIS CBD AND HEMP
It is only a matter of time before the US Federal government establishes a national policy and requirements for the industry. It is for your competitive advantage to be proactive and have the GMP for Cannabis CBD and Hemp certification in place so that you will be ready and in compliance when the FDA regulations come into effect.
Canada and many parts of Europe have already established regulations for GMP requirements. GMP for Cannabis CBD and Hemp requirements differ slightly between US GMP, EU GMP, and Canadian GMP. Among the different parts of the world that have their own codes, there are many common requirements. The quality management system for the Canadian cannabis industry is called GPP (Good Production Practice) and the quality management system for Europe for the Cannabinoid, CBD and Hemp industry called EU GMP.
COST OF GMP FOR CANNABIS CBD AND HEMP
The cost of GMP for cannabis CBD and hemp is based on the scope, complexity, and size of your business, the number of locations, the number of employees, and where your organization is in terms of your current quality management system related to GMP standards.
Our first onsite GMP consultation is free and after the initial visit, our experienced consultants will provide you with an estimate of the cost and time frame involved in the GMP for Cannabis CBD and Hemp implementation and certification process, Any further contract or commitment is required only if LMG is selected as the consultant to provide further services.
GMP FEES FOR CANNABIS CBD AND HEMP
The GMP for Cannabis CBD and Hemp fees can vary based on the scope of your business, location, and the amount of consultation you will require to successfully implement an effective quality management system tailored to your unique situation.
It is convenient and cost-effective for Cannabis, CBD, and Hemp manufacturers to integrate multiple quality management systems like GMP and ISO 9001. This will help them avoid developing and maintaining multiple lists of quality management system documents.
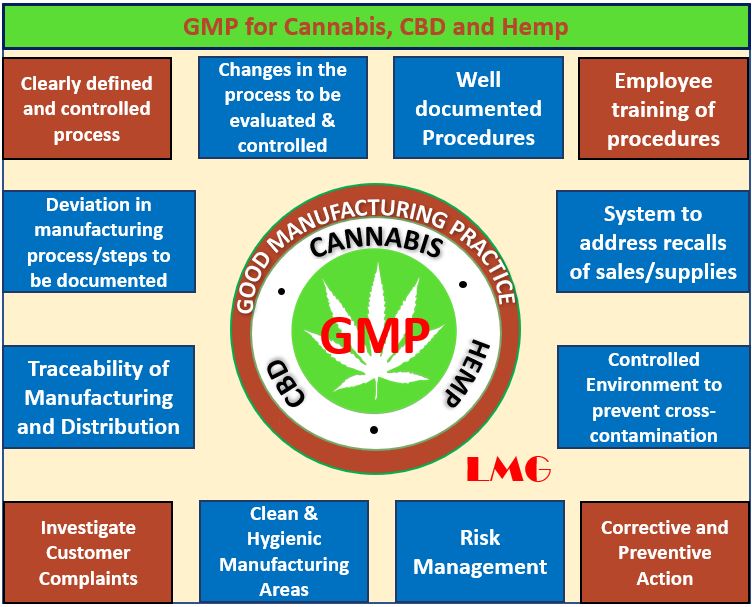
GMP REQUIREMENTS FOR CANNABIS CBD AND HEMP
- Products are consistently high in quality from batch to batch
- Products are used for their intended use
- Prevent any harm from occurring to the end-user
- Packaging and labeling requirements
- Process and Procedures to be documented.
- Traceability of Manufacturing and Distribution.
- Validation and verification of processes
- Standardized operating procedures (SOPs)
- Quality Management system requirements
- Sanitation and hygiene Manufacturing Requirements
- Testing requirements for chemical, physical, and biological contaminants
GMP PROCESS FOR CANNABIS CBD AND HEMP
GMP Process for Cannabis, CBD, and Hemp industry involves the following elements:
- GAP Analysis: Evaluation of your existing Quality Management System related to requirements of State and Federal Regulations (Title 21 CFR).
- Documentation & Records: Development of documents such as quality manual, procedures, forms, templates, etc.
- GMP awareness training: Training of your staff and management teams.
- SOP: Review of your Standard Operating Procedures
- Implementation: Implementing a well-documented Quality Management System throughout your organization.
- Internal Audit: identifying non-conformities related to GMP requirements.
- MRM: Management Review Meetings
- CAPA: Corrective and preventive actions /Root cause analysis addressing non-conformities.
- External Audit: FDA / Department of Health (DOH) audit support
- Non-Conformities: Closing of any non-conformities found. (FDA will issue an FDA 483 form with observations)
- Certification: Referral for Third-Party GMP Certification for State and Federal Cannabis, CBD and Hemp Programs.
GMP CONSULTANTS FOR CANNABIS CBD AND HEMP
Liberty Management Group (LMG) has a proven track record of successfully implementing and certifying various quality management systems such as GMP, ISO 9001, ISO 13485, CE Marking, etc. across the United States for over 14 years. Our experienced and competent GMP consultants for cannabis, CBD, and Hemp can assist you with successfully meeting all the quality management system requirements mandated by the State and Federal authorities.